Research Highlight: Hoffman
The Elusive 5′-Deoxyadenosyl Radical: Captured and Characterized by EPR and ENDOR Spectroscopies
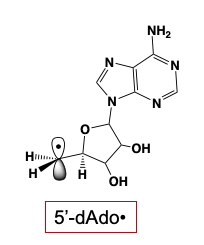
The 5'-deoxyadenosyl radical (5'-dAdo•) plays a central role in biology by initiating radical-based transformations through the abstraction of a substrate H-atom. This was first appreciated in studies beginning over sixty years ago, which showed that this radical is generated in reactions catalyzed by adenosylcobalamin-dependent (B12). The significance of the 5'-dAdo• radical in biology broadened dramatically with the recognition that it is also generated throughout the vast and diverse superfamily of radical S-adenosylmethionine (SAM) enzymes, which has over 100,000 identified members.
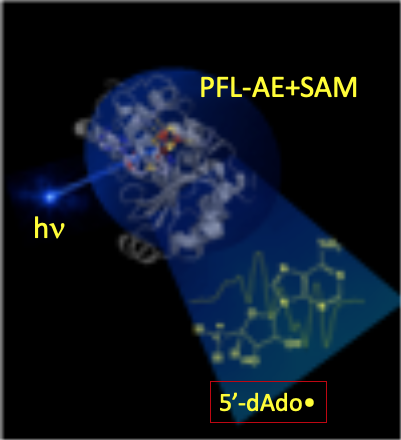
Notwithstanding the central biological role of 5'-dAdo•, for more than a half-century, numerous investigators had attempted to trap and characterize it without success: the radical was simply too reactive to trap! To our surprise, we discovered that irradiation of a radical-SAM enzyme (PFL-AE+SAM) with light of 450 nm, at cryogenic temperatures (~12 K) cleanly generated the 5'-dAdo•: the extraordinarily reactive, primary-carbon 5'-dAdo• radical had at last been captured. The electronic and geometric structures of this long-sought radical species were revealed through multifrequency electron paramagnetic resonance (EPR) and electron nuclear double resonance (ENDOR) spectroscopies, complemented with density-functional quantum-mechanical computation. Perhaps the most surprising finding about the radical’s properties is the absence of surprises: its remarkable reactivity accompanies properties that are almost precisely as foundational studies of organic radicals long ago would have predicted. We note that the method of generation and capture without radical rearrangement is itself as novel as the characterization is significant.