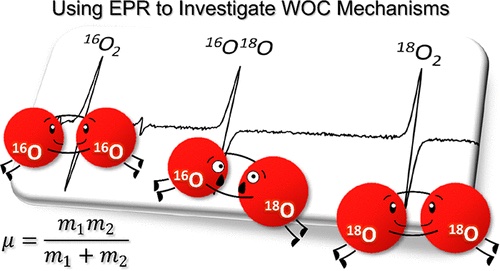
Electrocatalytic water oxidation is vital for converting solar energy into chemical fuels. Understanding the mechanisms of top electrocatalysts has been challenging. This study uses gas-phase EPR spectroscopy to quantify evolved dioxygen isotopologues, revealing water oxidation mechanisms on metal oxide electrocatalysts. Combining isotope fractionation with computational and kinetic modeling, the technique differentiates O–O bond-forming steps. Results show that for nickel-iron layered double hydroxide, a leading earth-abundant electrocatalyst, water oxidation occurs via dioxo coupling to form a side-bound peroxide, not through hydroxide attack to form an end-bound peroxide, aligning strongly with theoretical predictions. J. Am. Chem. Soc., 146, 22, (2024)
|
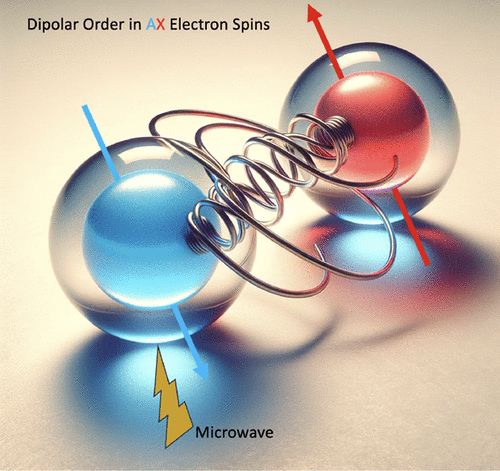
Coupled electron spin systems are crucial for applications like dynamic nuclear polarization (DNP), enhanced nuclear magnetic resonance (NMR), quantum information science (QIS), and studies of paramagnetic systems by electron paramagnetic resonance (EPR). However, characterizing these systems, especially at high magnetic fields, is challenging. This study examines a system with high concentrations of trityl radicals, showing a DNP enhancement profile for 1H NMR indicative of electron spin clusters. Selective microwave saturation in pump–probe Electron Double Resonance (ELDOR) experiments reveals electron spin hyperpolarization. This effect results from the formation of an out-of-equilibrium longitudinal dipolar order. The extent of hyperpolarization, influenced by tuning pulse length or interpulse delay, demonstrates that pump–probe ELDOR is effective for characterizing AX-type coupled electron spin systems, fundamental to DNP and quantum sensing. J. Phys. Chem. Lett., 15, 20 (2024)
|
|
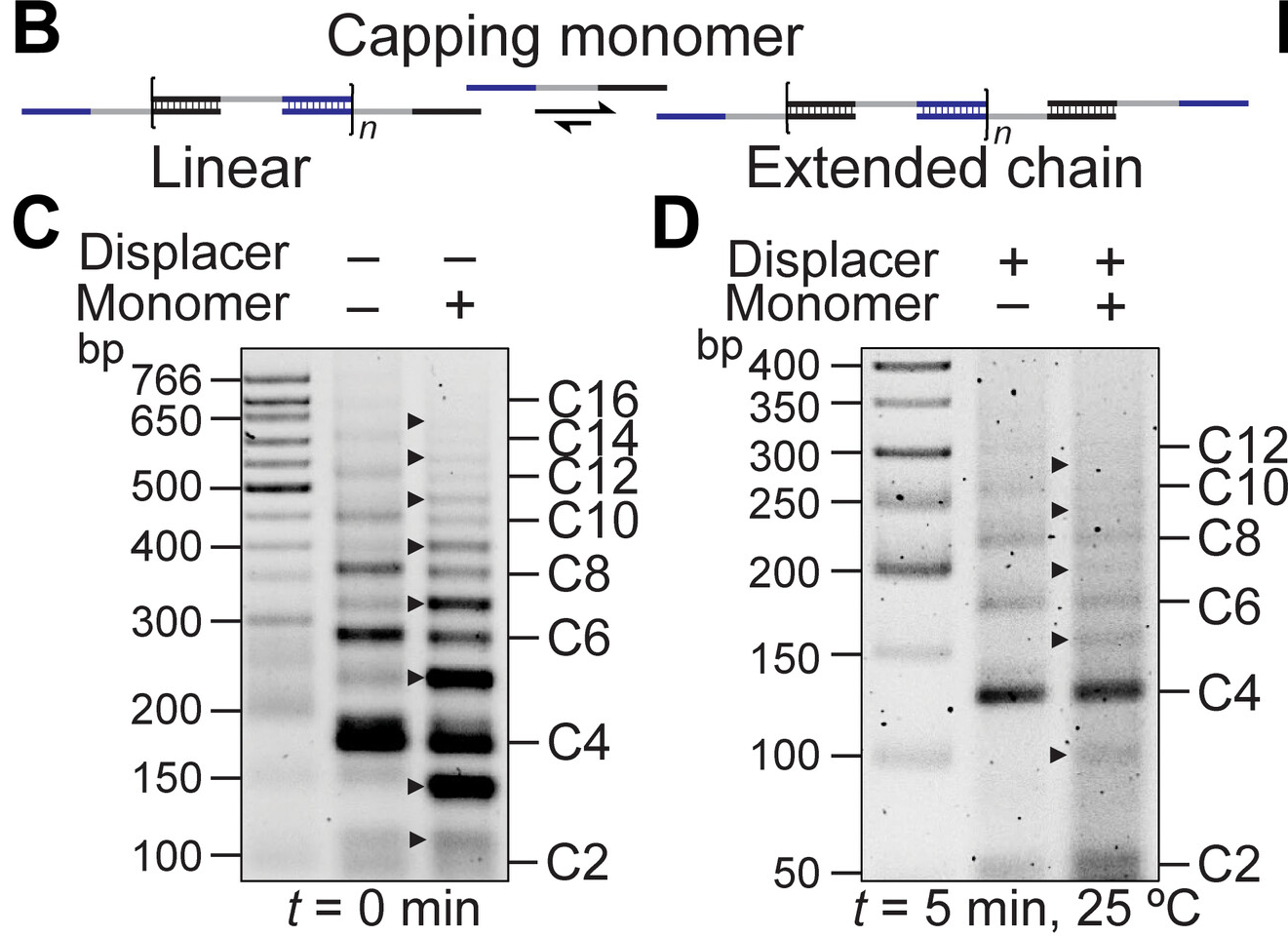 Molecular strain can influence chemical reactions but reversing a strain-promoted reaction after forming a thermodynamic product is difficult. This study presents a reversible, strain-promoted polymerization in cyclic DNA. Using single-stranded spacers as short as one nucleotide promotes DNA cyclization. Duplexing these spacers induces strain, causing ring opening and polymerization. Removing the duplexers triggers depolymerization and cyclic dimer recovery. This process remains reversible even with protein-conjugated DNA, allowing modulation between bivalent and polyvalent states. The findings highlight DNA's potential as a programmable ligand for creating shape-memory, self-healing, and stimuli-responsive materials. Sci. Adv. 10 (2024) Molecular strain can influence chemical reactions but reversing a strain-promoted reaction after forming a thermodynamic product is difficult. This study presents a reversible, strain-promoted polymerization in cyclic DNA. Using single-stranded spacers as short as one nucleotide promotes DNA cyclization. Duplexing these spacers induces strain, causing ring opening and polymerization. Removing the duplexers triggers depolymerization and cyclic dimer recovery. This process remains reversible even with protein-conjugated DNA, allowing modulation between bivalent and polyvalent states. The findings highlight DNA's potential as a programmable ligand for creating shape-memory, self-healing, and stimuli-responsive materials. Sci. Adv. 10 (2024)
|



 Molecular strain can influence chemical reactions but reversing a strain-promoted reaction after forming a thermodynamic product is difficult. This study presents a reversible, strain-promoted polymerization in cyclic DNA. Using single-stranded spacers as short as one nucleotide promotes DNA cyclization. Duplexing these spacers induces strain, causing ring opening and polymerization. Removing the duplexers triggers depolymerization and cyclic dimer recovery. This process remains reversible even with protein-conjugated DNA, allowing modulation between bivalent and polyvalent states. The findings highlight DNA's potential as a programmable ligand for creating shape-memory, self-healing, and stimuli-responsive materials.
Molecular strain can influence chemical reactions but reversing a strain-promoted reaction after forming a thermodynamic product is difficult. This study presents a reversible, strain-promoted polymerization in cyclic DNA. Using single-stranded spacers as short as one nucleotide promotes DNA cyclization. Duplexing these spacers induces strain, causing ring opening and polymerization. Removing the duplexers triggers depolymerization and cyclic dimer recovery. This process remains reversible even with protein-conjugated DNA, allowing modulation between bivalent and polyvalent states. The findings highlight DNA's potential as a programmable ligand for creating shape-memory, self-healing, and stimuli-responsive materials.